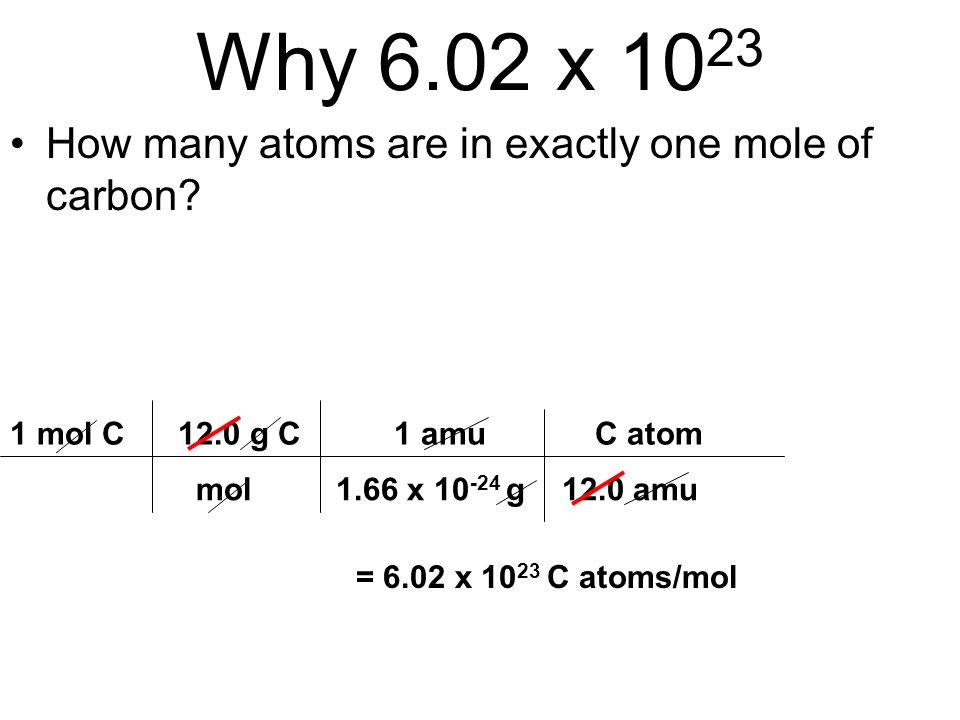
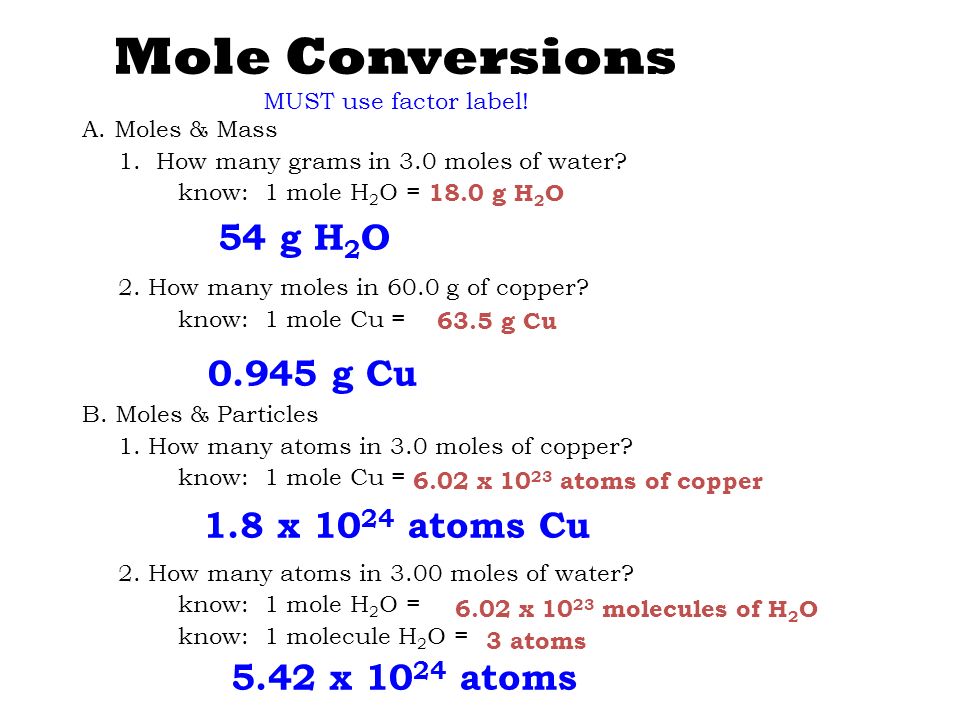
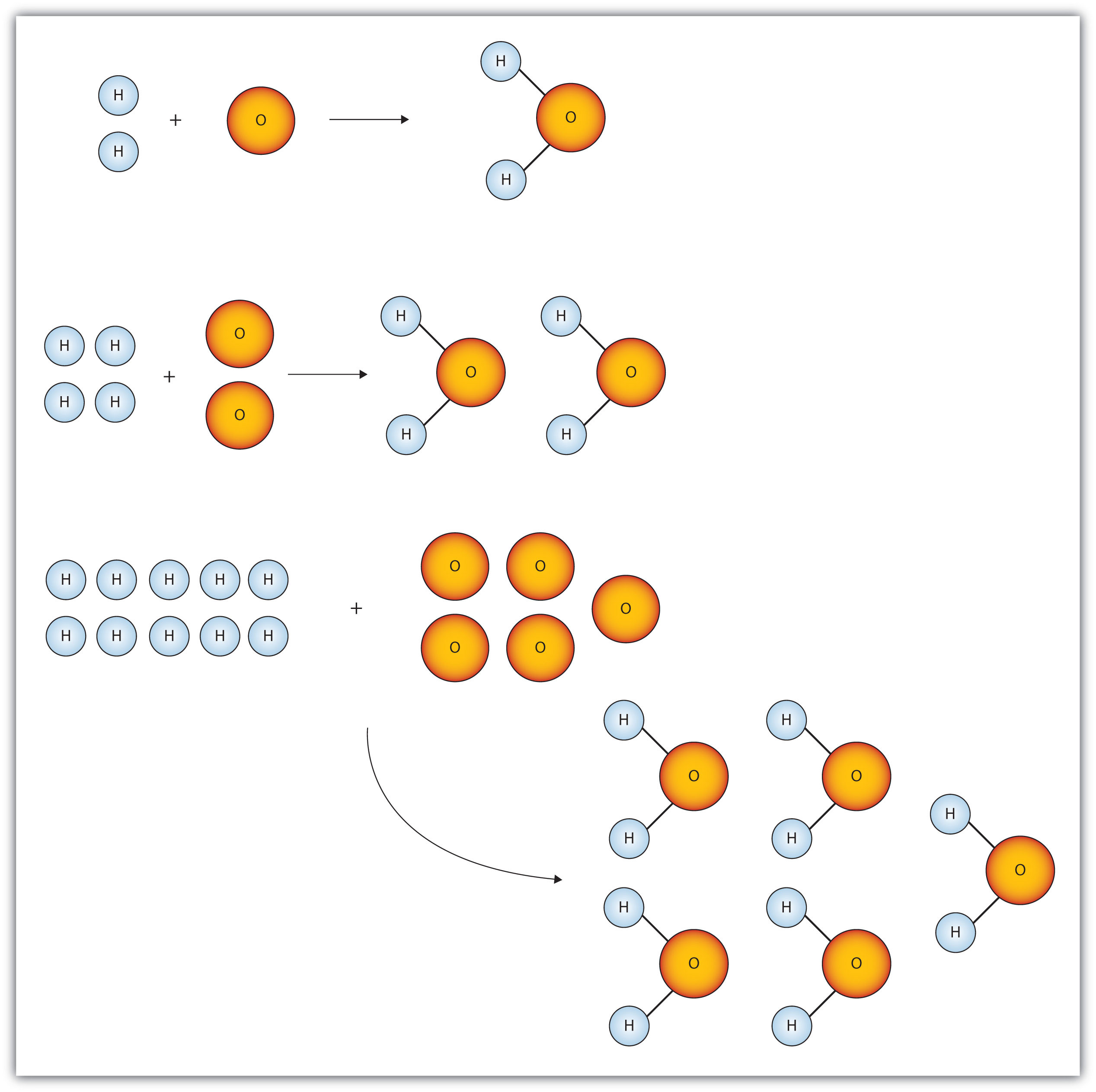
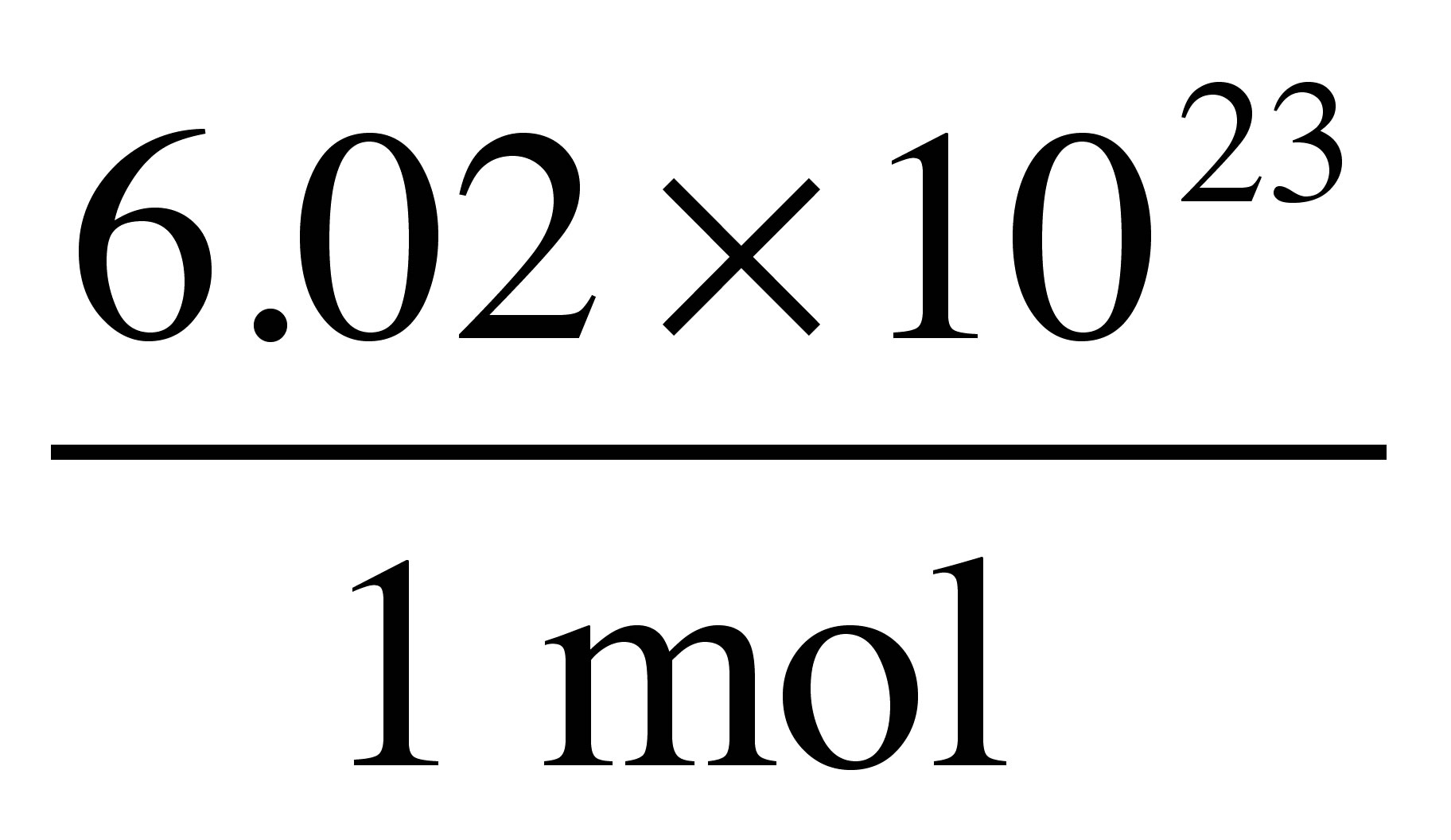The Mole & Chemical Quantities. The Mole Mole-the number of particles equal to the number of atoms in exactly 12.0 grams of carbon mol = 6.02 x. - ppt download

Chemistry Warm Up: Mole / Mass / Particles 1.What is the mass of one mole of water? 2.If one milliliter of water has a mass of 1.00grams, how many moles. - ppt download